DAR1 Antibody-Drug Conjugates
DAR1 ADC – Next-Generation Low-DAR Antibody-Drug Conjugates
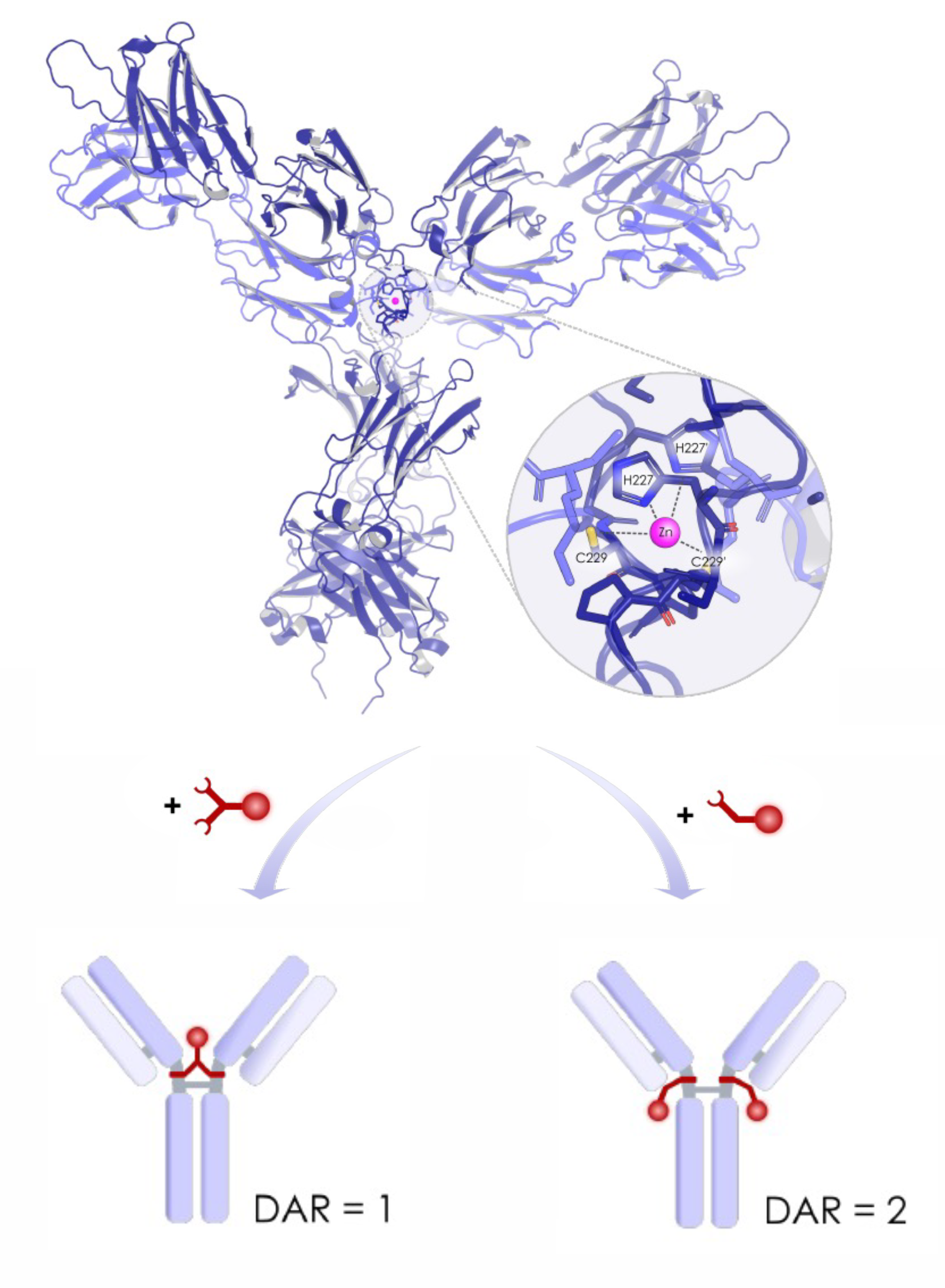
DAR1 antibody-drug conjugates are emerging as a strong alternative to conventional high-DAR designs. With a single payload attached per antibody, DAR1 architectures reduce hydrophobicity, improve developability, and often show cleaner pharmacokinetics. Syndivia’s GeminiMab platform builds on these advantages by using a hinge-conjugated, site-specific DAR1 design that supports high-dose administration without compromising stability or antitumor activity.
Why DAR1 ADCs Matter
Most ADCs rely on DAR2–DAR8 formats, which can trigger aggregation, rapid clearance, and dose-limiting toxicity. DAR1 ADCs minimize these liabilities:
lower hydrophobicity
improved serum stability
predictable PK
lower off-target toxicity
wider therapeutic window
Clinical and preclinical evidence increasingly shows that DAR1 ADCs can match or outperform higher-DAR constructs when the linker-payload chemistry is optimized, the conjugation is restricted to the hinge region to avoid diminishing antibody activity, and the antibody remains stable at high dose.
GeminiMab – A DAR1 Platform Built for High Dose
Syndivia’s GeminiMab platform introduces a hinge-conjugated DAR1 architecture that maintains antibody integrity and delivers strong efficacy, even in low-expressing tumors. Key features include:
Single, controlled conjugation site for reproducibility
Reduced hydrophobicity enabling mg/kg-level dosing
High serum stability via optimized conjugation chemistry
Consistent activity across high- and low-target expression models
Improved manufacturability due to cleaner biophysical properties
GeminiMab has demonstrated potent in vivo responses across several targets while enabling safe, predictable dose escalation beyond 10 mg/kg in humans.
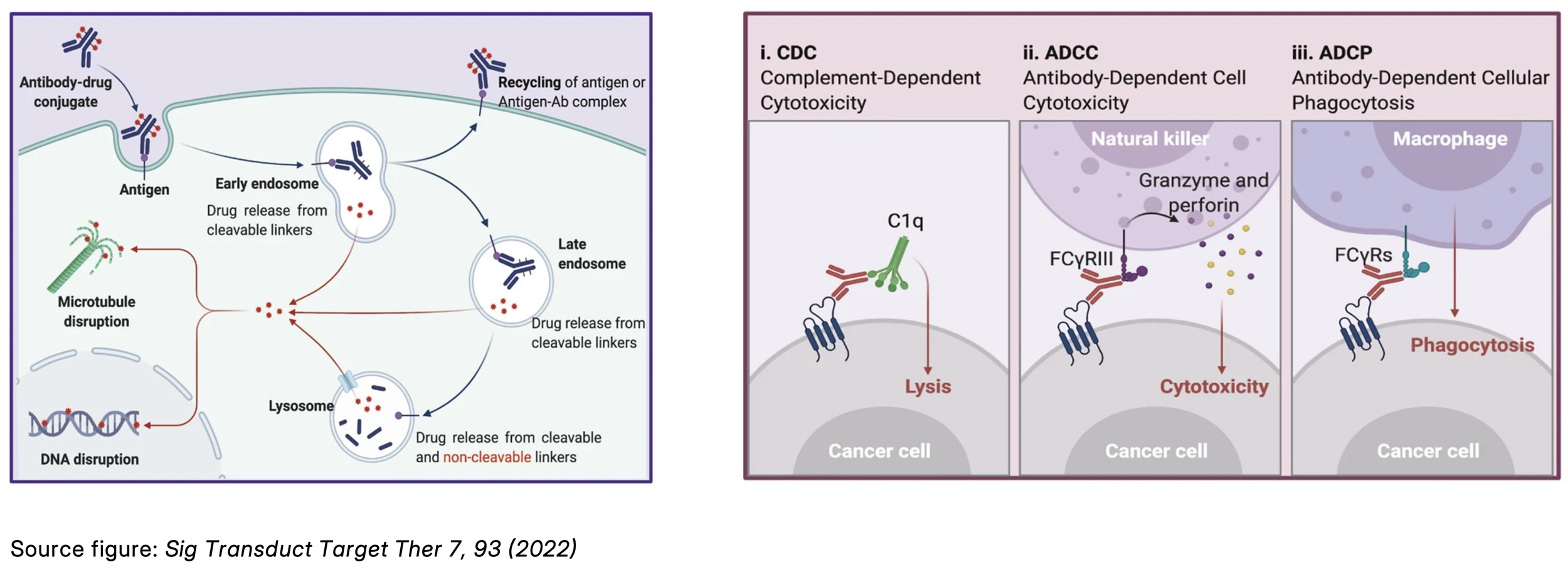
Higher dosing unlocks the full ADC mechanism
At higher, yet still tolerable doses, ADCs can fully leverage both components of their mechanism of action:
Antibody-driven immune effects (ADCC, ADCP, CDC)
Payload-driven cytotoxicity via internalization and endosomal release
This dual mechanism amplifies efficacy by preserving Fc-mediated antitumor functions while maintaining strong payload potency:
Applications
The DAR1 format is well suited for:
high- and low-expression tumor antigens
payloads requiring strict exposure control
programs where ADC hydrophobicity or aggregation have been blocking development
Syndivia is advancing a portfolio of early DAR1 ADC programs for undisclosed targets.